"Failure Analysis of Materials - An Introduction" by T.D. Burleigh (2018), ISBN: 978-1-387-45720-5, available at
www.Lulu.com.
This textbook covers the important steps in conducting a failure analysis, without boring the student to death. A material failure is defined as a part breaking unexpectedly. The part can be metal, plastic, ceramic, or glass, and by breaking we mean that there is a fracture face or damaged surface to examine. Failure analysis is the science of determining how and why the part broke. An accurate failure analysis is key to making a better product. If one does not understand why a part failed, the it is only guesswork as how to fix it. Failure analysis of materials is a multi-disciplinary field because it requires people skills in asking the right questions, engineering skills in calculating the stresses, and metallurgical skills in understanding the alloys and interpreting the micrographs. The final skill is writing a comprehensive report. These topics and more are covered in this book.
|
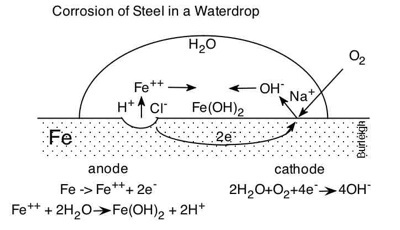
"Corrosion of Steel in a Waterdrop" may been seen at
http://www.youtube.com/watch?v=d0LUB90uDaEm
This video is a series of time-lapse micrographs taken by Dr. Burleigh in 2011. These timed micrographs show a water drop containing pH indicator solution, sitting on the surface of bare steel. The water drop is Socorro, NM, municipal water, containing many dissolved minerals including 14 ppm NaCl. As time progresses, the color of the pH indicator solution changes, indicating that acidic and caustic regions are developing in the water drop. Dr. Burleigh narrates the micrographs and explains the corrosion phenomena witnessed in the video.
|
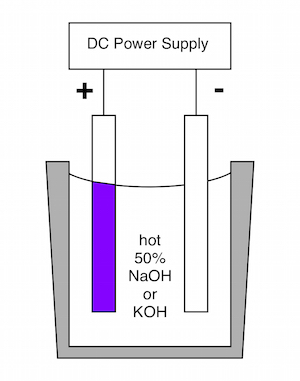
"How to Anodize Steel" may been seen at
https://www.youtube.com/watch?v=7g-azzYnMYo
This 2015 video describes the methods developed by Prof. Burleigh and his students for anodizing steel in hot caustic electrolytes. This process is the subject of two publications. The anodized layer is a nano-porous magnetite film that is adherent to the surface. The reflection of light from the top and bottom surfaces of the oxide film create a dichromic color that changes with viewing angle.
T.D. Burleigh, P. Schmuki and S. Virtanen, "Properties of the Nanoporous Anodic Oxide Electrochemically Grown on Steel in Hot 50% NaOH." Journal of the Electrochemical Society, (Jan 2009), 156, 1, C45-C53.
JECS_2009
T.D. Burleigh, T.C. Dotson, K.T. Dotson, S.J. Gabay, T. Sloan, S.G. Ferrell, "Anodizing Steel in KOH and NaOH Solutions," Journal of the Electrochemical Society (Oct. 2007), 154, 10, 579-586.
JECS_2007
Related website:
www.steelanodize.corrosionhelp.com
|

"Corrosion of Aluminum Alloys" may be seen at
http://youtu.be/YeFTEzeX56k
This educational video (produced by Dr. Burleigh in 2014) is a brief introduction into the corrosion of aluminum alloys. The video shows the common forms and the theoretical mechanisms of aluminum corrosion. This video was funded by a grant from Modern Light Metals.
|
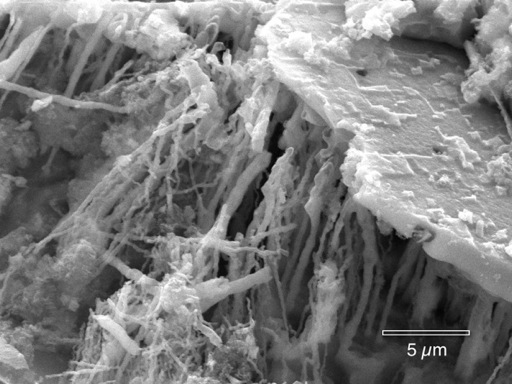
Microbes inside a corrosion pit in copper, from Thomas D. Burleigh, Casey G. Gierke, Narjes Fredj and Penelope J. Boston, "Copper Tube Pitting in Santa Fe Municipal Water Caused by Microbial Induced Corrosion," Materials (2014), 7, p. 4321-4334.
http://www.mdpi.com/1996-1944/7/6/4321.
|

Multi-colored Cu2O films deposited on copper. From
N. Fredj and T.D. Burleigh, "Transpassive Dissolution of Copper, and Rapid Formation of Brilliant Colored Copper Oxide Films," Journal of the Electrochemical Society (2011), 58, 4, C104-C110.
(Fredj-Burleigh-2011.pdf)
|

1010 steel sheet anodized in one minute steps. From Burleigh et al, "Anodizing Steel in KOH and NaOH
Solutions," Journal of the Electrochemical Society, 154, 10, p. C579-586
(JECS_2007.pdf).
For more information, see also www.steelanodize.com and JECS_2009.pdf.
|

Eighteen tuning forks machined from seventeen different alloys and one polymer, are used as classroom
demos. Each fork has its own unique resonant pitch and harmonics, dampening, weight, color and stiffnesss.
A two-page article has been published entitled, "Tuning Forks for Vibrant Teaching." Journal of Metals (2005), 57, 11, 26-27.
(TuningJOM2005.pdf) It may aslo be
found on the
Journal of Metals website.
|
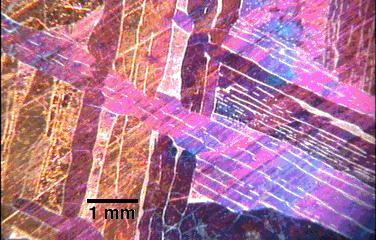
The polished, etched and heat-tinted face of the Gibeon Meteorite shows a crystal
pattern denoting a cooling rate of 1 C per million years (from the Materials and Metallurgical Engineering
Department Brochure).
|

A schematic of the photoelectrochemical apparatus used for measuring photocurrents and
photovoltages on metals immersed in a liquid. J.R. Birch and T.D. Burleigh,
"Oxides Formed on Titanium by Polishing, Etching, Anodizing, or Thermal Oxidizing," Corrosion (2000), 56, 12, 1233-1241.
(BirchBurleigh2000.pdf)
|
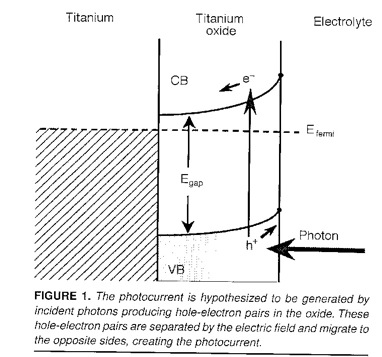
The photocurrents are result from light exciting electrons in the oxide film, in the presence of a
Schottky barrier. The electrons are excited from the valence band (V.B.) to the conduction band (C.B.)
where they flow down hill under the influence of the electric field. The electric field is a result of the
mismatch of the Fermi levels of the electrolyte and the metal.
J.R. Birch and T.D. Burleigh, "Oxides Formed on Titanium by Polishing, Etching, Anodizing, or
Thermal Oxidizing," Corrosion (2000), 56, 12, 1233-1241.
(BirchBurleigh2000.pdf)
|
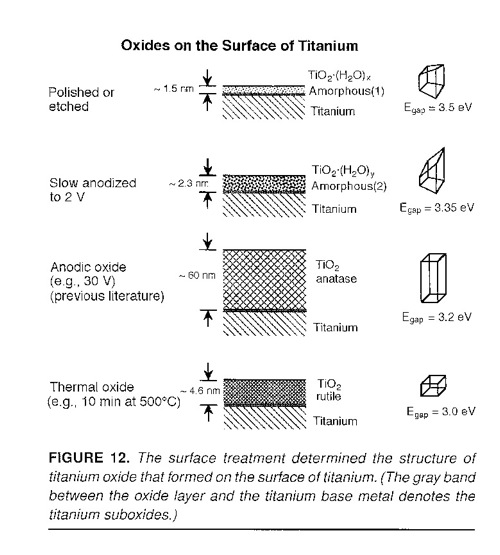
The photocurrents may be used to identify the crystal structure of the titanium oxide since the different oxides
have different bandgaps.
J.R. Birch and T.D. Burleigh, "Oxides Formed on Titanium by Polishing, Etching, Anodizing, or
Thermal Oxidizing," Corrosion (2000), 56, 12, 1233-1241.
(BirchBurleigh2000.pdf)
|
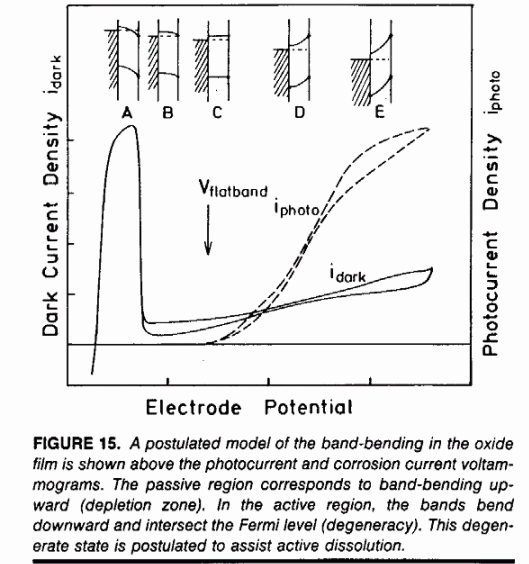
The active-passive transition of may be modeled as a semiconductor film that becomes degenerate at
high or low potentials. During degeneracy, the conduction or valence bands bend across the Fermi level and the
oxide becomes an electric conductor. from T.D. Burleigh, "Anodic Photocurrents and Corrosion Currents on
Passive and Active-Passive Metals," Corrosion (1989), 45, 6, 464-471
(Corrosion1989.pdf)
|
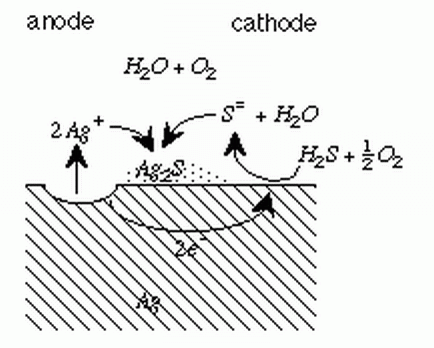
The tarnishing of silver requires an atmosphere containing hydrogen sulfide, oxygen and water vapor.
A electrochemical mechanism is proposed for the tarnishing of silver.
T.D. Burleigh, Y. Gu, G. Donahey, M. Vida, D.H. Waldeck,
“Tarnish Protection of Silver using a Hexadecanethiol Self-Assembled Monolayer and Descriptions of
Accelerated Tarnish Tests,” Corrosion (2001), 57, 12, 1066-1074.
(BurleighWaldeck2001.pdf)
|
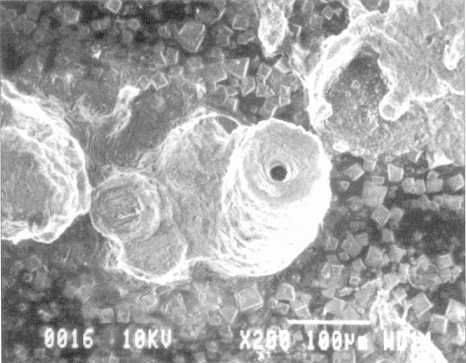
A corrosion chimney forms above the corroding pit on aluminum corroding in saltwater.
T.D. Burleigh, E. Ludwiczak, and R.A. Petri, "Intergranular Corrosion of an Al-Mg-Si-Cu Alloy,"
Corrosion (1995), 51, 1, 50-55. (Corrosion1995.pdf)
|
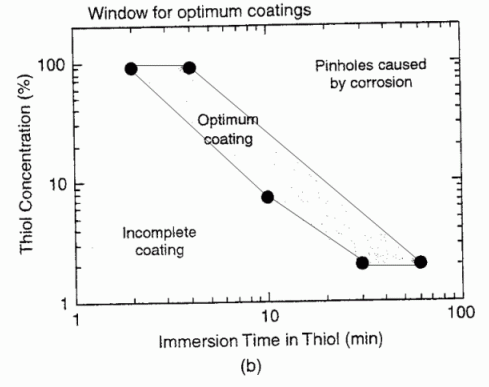
Silver may be protected from tarnishing by a self-assembled monolayer (SAM) of hexadecanethiol. The SAM is
prepared by cleaning, etching, rinsing, then immersion in a thiol solution for a certain time period. Too short of time
leads to an incomplete film, and too long of time leads to pinhole corrosion.
Figure 10b from
T.D. Burleigh, Y. Gu, G. Donahey, M. Vida, D.H. Waldeck,
“Tarnish Protection of Silver using a Hexadecanethiol Self-Assembled Monolayer and Descriptions of
Accelerated Tarnish Tests,” Corrosion (2001), 57, 12, 1066-1074.
(BurleighWaldeck2001.pdf)
|
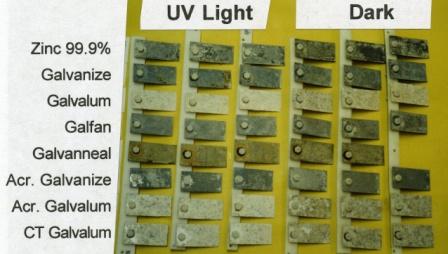
Zinc corrodes faster under UV illumination. E.A. Thompson and T.D. Burleigh,
"Accelerated Corrosion of Zinc Alloys Exposed to Ultraviolet Light," Corrosion Engineering,
Science and Technology (2007), 42, 3, p. 237-241.
(Zinc&UV_2007.pdf).
|
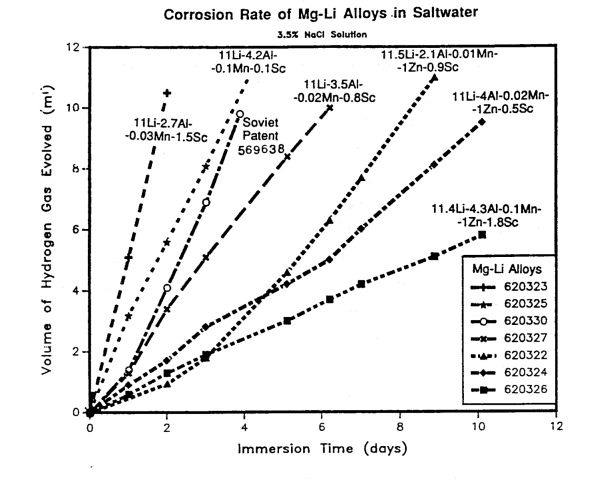
A corrosion resistant Mg-Li alloy is made by alloying with scandium.
T.D. Burleigh, R.K. Wyss, "Dual Phase Magnesium Based Alloy having Improved Properties,"
U.S. Patent No. 5,059,390 (October 22, 1991).
(MgLiSc.pdf)
|
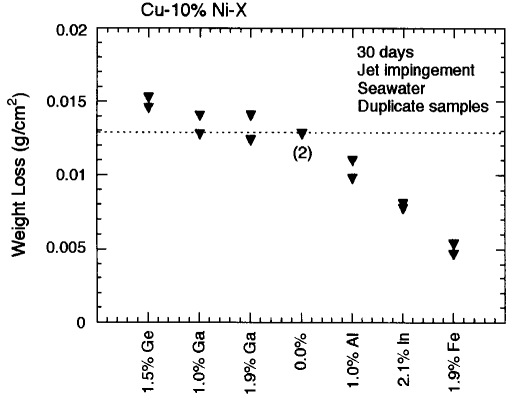
Improvement in the erosion corrosion resistance of Cu-10%Ni was achieved by adding indium.
T.D. Burleigh and D.H. Waldeck, "Effect of Alloying on the Resistance of Cu-10% Ni Alloys to
Seawater Impingement," Corrosion (1999), 55, 8, 800-804.
(Corrosion1999.pdf)
|